安全性数据则是“三联方案”的又一个加分项 , 两组的3-5级不良事件发生率、因不良事件停药的患者比例、因不良事件死亡的患者比例都基本一致 ,加用帕博利珠单抗还是安全可控的 。
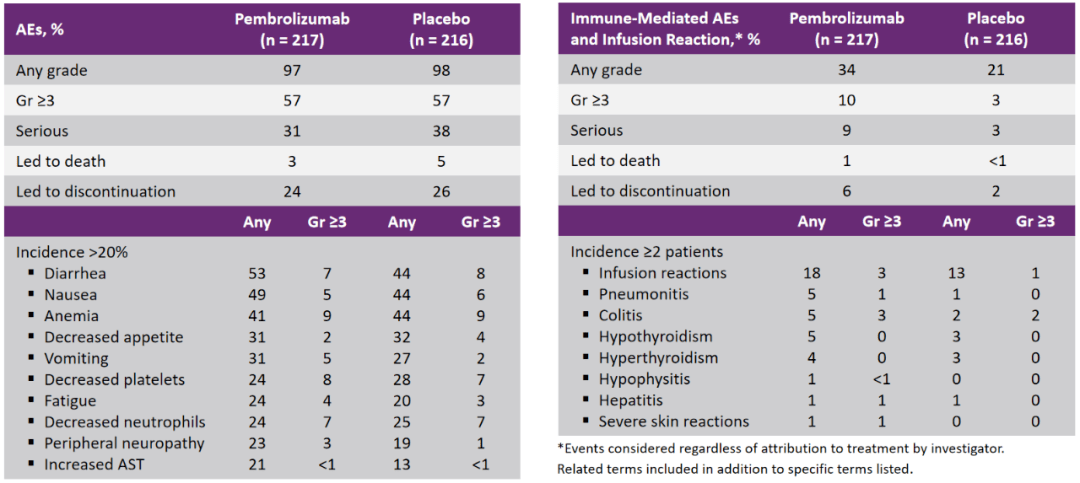
文章图片
研究安全性数据汇总
总之 , 基于KEYNOTE-811研究的初步疗效数据 , 美国FDA在2021年5月份 , 就已加速批准帕博利珠单抗联合曲妥珠单抗+化疗 , 一线治疗HER2阳性胃/胃食管连接癌的适应证 。 这到底是精准超前 , 还是有些大胆冒失 , 就要看未来的结果啦 。
参考文献:
1.Janjigian Y Y, Kawazoe A, Ya?ez P, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer[J]. Nature, 2021.
2.Mitani S, Kawakami H. Emerging targeted therapies for HER2 positive gastric cancer that can overcome trastuzumab resistance[J]. Cancers, 2020, 12(2): 400.
4.Chaganty B K R, Qiu S, Gest A, et al. Trastuzumab upregulates PD-L1 as a potential mechanism of trastuzumab resistance through engagement of immune effector cells and stimulation of IFNγ secretion[J]. Cancer Letters, 2018, 430: 47-56.
5.Janjigian Y Y, Maron S B, Chatila W K, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial[J]. The Lancet Oncology, 2020, 21(6): 821-831.
6.Rha S Y, Lee C-K, Kim H S, et al. Targeting HER2 in combination with anti-PD-1 and chemotherapy confers a significant tumor shrinkage of gastric cancer: A multi-institutional phase Ib/II trial of first-line triplet regimen (pembrolizumab, trastuzumab, chemotherapy) for HER2-positive advanced gastric cancer (AGC)[J]. Journal of Clinical Oncology, 2020, 38(15_suppl): 3081.
本文作者丨谭硕
- 那过|孕期吃药对胎儿有没有影响
- 失眠者|长期失眠等于慢性自杀!医生建议:6种食物多吃点,帮你找回睡意
- 诺西那生|“庆幸自己生在这个未来可期的时代!”
- 癌症|年仅四十出头却确诊癌症晚期,现在他只想说……
- 妊娠期|安医大四附院多学科合力挽救一名重度子痫孕妇
- 健康|寒假,健康不放假!
- 商品|北京疾控:国外疫情高发期间尽量减少购买境外商品
- 冬季是女性减肥的黄金时期,多吃1元蔬菜,少油热量低,瘦身快
- 商品|北京疾控:国外疫情高发期尽量减少购买境外商品,收取境外邮件要注意这些
- 阳性|北京病例自述近期曾收发过国际邮件,经核酸检测现阳性
